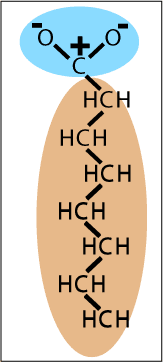
| Oils are members of a class of molecules that don't mix well with water. Oils are hydrocarbon chains containing about ten carbon atoms. The middle sections of two oil molecules are drawn on the right. Oil molecules bind to each other like water molecules do, but with much weaker forces because there are no significant electronic charges on the atoms.
If the chains are only 1, 2, 3, or 4 carbons long, methane, ethane, propane or butane, the molecules bind to each other so weakly that they form gases at room temperature. If the molecules are 20 carbon atoms long they bind so strongly as to form waxes. In between these two classes of hydrocarbons are the oils. As you might imagine there are variations on this theme. If some of the hydrogen atoms are missing on adjacent carbons they can form "double bonds", and these oils are called "unsaturated". As you guessed, oils with only single bonds are called "saturated".
Thus molecules that are non-ionic and don't mix with water are hydrophobic while molecules that are ionic do mix well with water are thus called hydrophilic.
|
|
 |
|